Dáil membership to increase


New population figures released by the CSO indicate that the overall ratio of citizens to TDs has exceeded constitutional parameters.
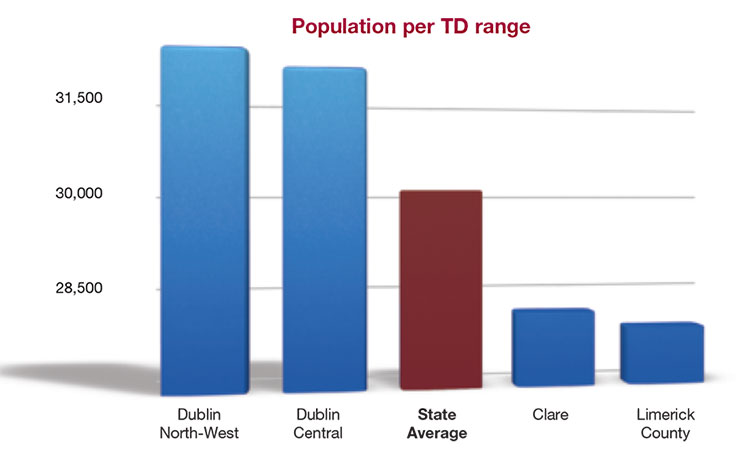
Preliminary findings of Census 2016 have established that, of 40 constituencies across the State, 25 have now breached the legal limit for representation as determined by Article 16 of the Constitution. This stipulates that the total Dáil membership must not be less than one member for each 30,000 individuals. Population increases since 2011 mean that the average number of people represented by each of the 158 Dáil members currently stands at 30,114 which amounts to constitutional underrepresentation.
Consequently, a Constituency Commission has been established by Minister for the Community and Local Government Simon Coveney. The five-member body is composed of a High Court judge, the clerks of both the Dáil and the Seanad, the Ombudsman and the Secretary General of the Department of the Environment, Community and Local Government.
Senior statistician with the CSO Deirdre Cullen comments: “[The commission] will begin their deliberations in redrawing the constituencies. Clearly there will have to be at least one more TD.” Following the publication of the CSO’s final census report later this year, the commission will be afforded a period of three months in which to produce a report for the Ceann Comhairle.
While there will be a minimum requirement for the election of at least 159 TDs, it is anticipated that, following constituency boundary restructuring, the commission will make recommendations for an increase in Dáil membership by a maximum of two. This reshuffle comes in the wake of recent boundary adjustments which condensed constituency numbers from 43 to 40 and TDs from 166 to 158 ahead of the 2016 general election. The last increase in Dáil seats occurred in 1981 when the first Constituency Commission upped the figure from 147 to 166. In 2011 the Fine Gael government pledged to cap TD numbers at their lowest level since the 1981 election.
The three constituencies which most greatly exceed the constitutional upper limit are Dublin North-West (32,299), Dublin Central (32,016) and Dublin Rathdown (31,375). Limerick County is currently the constituency with the lowest population per TD (27,916).